Elucidating the role of effector proteins in cell cycle progression
A key step in the eukaryotic cell cycle is the G1 to S phase transition and this step is tightly coupled to the transcriptional control of genes involved in growth and DNA replication.
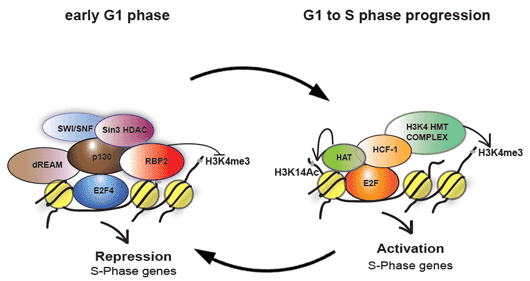
Figure 1. Proposed model for activation and repression of S-phase promoters. . During G1/S phase transition, HCF-1 and its associated H3K4 HMTs are recruited to E2F-responsive promoters, replacing the pRb repressor complex. The E2F-HCF-H3K4HMT complex then deposits the H3K4 tri-methylation mark and activates transcription of S-phase genes. In early G1 phase, E2F4 and p130 recruit demethylase RBP2 to erase the H3K4 tri-methylation mark and repress these promoters. Hence H3K4 tri-methylation mark is dynamically regulated during the cell cycle.
In mammalian cells, the E2F family of transcription factors primarily controls this temporal gene expression. We had previously shown that HCF-1 is an important regulator of G1 to S-phase transition and plays a direct role in the activation of E2F-responsive promoters through the cell-cycle-specific recruitment of the Mixed Lineage leukemia (MLL)-family of Histone H3 lysine 4 (H3K4) histone methyltransferases. However, how and when the H3K4 tri-methylations marks are reset during the cell cycle was not known. Our recent work shows that E2F4 and pocket protein p130 recruit the demethylase KDM5A/RBP2 to these promoters in early G1 to erase the H3K4 tri-methylations marks and repress the E2F-responsive promoters (Figure 1). While this work has added new effectors, how E2F-responsive promoters are regulated is still poorly understood. In our laboratory, we aim to discover new effector proteins involved in regulation of E2F-responsive promoters and better understand how these effectors influence the chromatin modulation during cell cycle progression.
|