Projects
Overview of Cell Signalling and an Introduction to Inositol Pyrophosphates
The principal mechanisms by which cells communicate with each other are conserved across biological systems, from prokaryotes to unicellular eukaryotes to mammals. When a cell receives a signal from its extracellular environment or from other cells, it triggers a cascade of biochemical processes collectively termed signal transduction. This results ultimately in a cellular response to the signal that initially triggered the cascade. For example, when you eat, the carbohydrates in your food are broken down to glucose, which enters your bloodstream. Glucose (the signal) then enters beta cells in your pancreatic islets, where it triggers a signal transduction pathway that results ultimately in the release of insulin from the pancreas into your bloodstream (the response). Insulin in turn (now the signal), binds to insulin receptors on the cells of your skeletal muscles, triggering a signalling cascade that results in glucose being transported from the blood into the skeletal muscle cells (the response).
Broadly, our research interests span the study of signal transduction in biological systems. Specifically, we are interested in understanding the role of inositol pyrophosphates in eukaryotic physiology and metabolism. The inositol pyrophosphates, diphosphoinositol pentakisphosphate (PP-IP5 or IP7) and bisdiphosphoinositol tetrakisphosphate ([PP]2-IP4 or IP8), shown in Figure 1, contain high energy pyrophosphate or diphosphate bonds, and participate in diverse cellular activities. IP7 is synthesised from inositol hexakisphosphate (IP6) by IP6 kinases, and IP7 kinases convert IP7 to IP8. Saccharomyces cerevisiae have a single IP6 kinase, called KCS1, whereas mammals have three isoforms, IP6K1, IP6K2 and IP6K3. Inositol pyrophosphates transfer their beta phosphate group to pre-phosphorylated serine residues on proteins to form pyrophosphoserine (Figure 2). This novel modification, pyrophosphorylation, occurs on several proteins within the cell, including proteins involved in ribosome biogenesis and vesicular trafficking. Bakers yeast (Saccharomyces cerevisiae) that lack inositol pyrophosphates display defects in growth, telomere length maintenance, vesicular trafficking, stress response, and phosphate homeostasis. Transgenic mice with low levels of inositol pyrophosphates have lower body weight than wild type mice, display low insulin levels and defective spermatogenesis. Recent work has revealed that inositol pyrophosphates regulate insulin release and apoptosis, with an implicit link to diabetes and cancer. It is now believed that inositol pyrophosphates mediate these diverse biological functions either by specific binding to proteins, or via protein pyrophosphorylation.
In our laboratory, we aim to understand the biochemical link between protein pyrophosphorylation and cellular phenomena regulated by inositol pyrophosphates. We have begun by utilising S. cerevisiae and mammalian cell lines as model systems to investigate the signalling pathways that are altered when inositol pyrophosphate levels are perturbed. Our long term goal is to understand the mechanisms by which inositol polyphosphates mediate cellular events that are relevant to human physiology and disease.
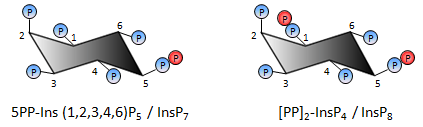
Figure 1. Inositol pyrophosphates. Inositol polyphosphates are small biological molecules that contain multiple phosphate moieties linked to inositol. The inositol polyphosphates that contain ' high energy ' pyrophosphate or diphosphate groups (shown in red) are called diphosphoinositol polyphosphates, or more simply, inositol pyrophosphates. The two most abundant inositol pyrophosphates found in eukaryotes are diphosphoinositol pentakisphosphate (InsP7) and bis-diphosphoinositol tetrakisphosphate (InsP8).
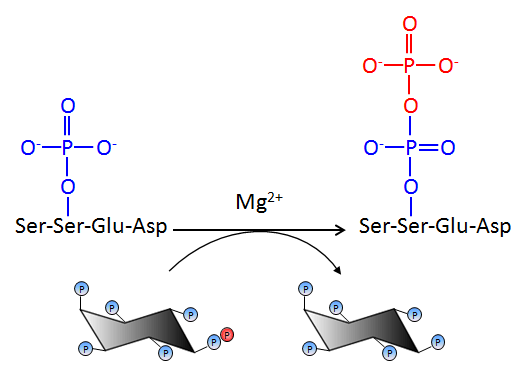
Figure 2. Protein pyrophosphorylation. Inositol pyrophosphates such as InsP7 transfer their beta phosphate group to a pre-phosphorylated serine residue to generate pyrophosphoserine. Pyrophosphorylation occurs in the presence of divalent magnesium ions, and only on serine residues flanked by acidic amino acids. |